A team of researchers at the Harvard School of Engineering and Applied Sciences that is headed by Sriram Ramanathan is working on developing fuel cells. If Ramanathan is to be believed, the solid-oxide fuel cells the visionary and specialist in the field is making along with other scientists, will become a highly sought after technology in days to come.
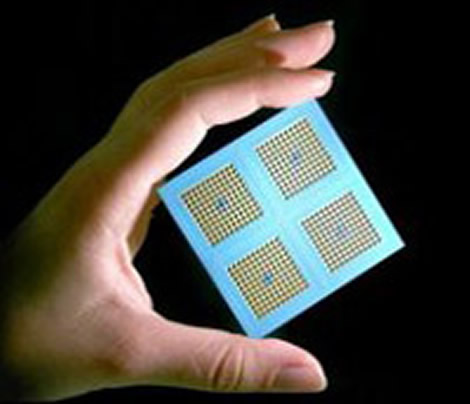
How will solid-oxide fuel cells be generated?
The solid-oxide fuel cells that are capable of replacing fossil fuel with pollution less fuel are generated with the use of the plentiful fuel resources and low operating temperatures, along with some material that is of low cost, and some other small devices.
How do solid-oxide fuel cells work?
In their research paper that Ramanathan published along with Bo Kuai Lai, a Research Associate at SEAS, and Ph.D. candidate Kian Kerman, they have explained how they oxidize the fuel so that a current of electrons can be generated back towards the cathode. This fuel is oxidized through oxygen ions that travel towards the anode from the cathode through the electrolyte. In this way chemical energy is converted into an electric current to make fuel cells function.
New research to make solid-oxide fuel cells available
Though even they realize that making fuel cells practical and affordable is going to take some time, the two studies that have been printed in the Journal of Power Sources recently, the research team of Ramanathan has pointed out that the technology may reach the market earlier than expected due to advancement in solid-oxide fuel cells technology.
THE TWO STUDIES ON SOLID-OXIDE FUEL CELLS
The first study on solid-oxide fuel cells
As Ramanathan and his team had also showed in practical application also, the paper talked of operational and steady SOFCs or films that utilize very thin but tightly-packed layers of special ceramic films to reduce electrolyte by hundreds and thousands of time.
The main problem while using the all-ceramic thin-film SOFCs was that they had to be contained in platinum electrodes. This made them unreliable and costly. To overcome this problem, Ramanathan’s team has come up with platinum-free micro- solid-oxide fuel cells. These cells are not only more reliable but also low in cost.
The second study on solid-oxide fuel cells
Ramanathan and his team realized the fact that in most cases, solid-oxide fuel cells can only be used for stationary power generation because they function at a very high temperatures ranging between 800-1000º Celsius. But in their second research paper published recently, Ramanathan and his team has shown that methane-fueled micro SOFC can function at less than 500º C. Now scientists want to make methane-fueled micro SOFC work at around 300º C.
Gases used to fuel solid-oxide fuel cells
Methane is being preferred to fuel solid-oxide fuel cells because apart from being reliable and affordable in terms of finance, there is no scarcity of it. It can also be more useful in low temperature. Though researches have tried to replace methane with hydrogen, this experiment failed because, since it requires more processing, hydrogen proves to be a costlier gas for this purpose.
Advantages of fuel cells operating at low temperature
- It makes the material more reliable.
- It reduces cost.
- The start-up time becomes less.
- It makes the use of solid-oxide fuel cells in diverse fields such as portable electrons and transport services.
Drawbacks of solid-oxide fuel cells
- Though solid-oxide fuel cells are a good source of fuel, they leave water as waste.
- Since using solid-oxide fuel cells at a low temperature still remains a distance dream, you cannot charge laptops and phones or use them as fuel for cars and trucks.
- The use of solid-oxide fuel cells is still at experimental stage. Though it has proved its benefits in laboratory, a serious practical use is still awaited.
Ramanathan’s hard work is going to pay-off soon and the technology of solid-oxide fuel cells will be brought to extensive use in times to come.
 Follow
Follow